我室吴川六教授在Angew. Chem. Int. Ed.上发表题为“Precisely Regulated and Efficient Locking of Linear Peptides into Stable Multicyclic Topologies through a One-Pot Reaction”的研究论文。
文章链接:http://onlinelibrary.wiley.com/doi/10.1002/anie.201610942/full
摘要:
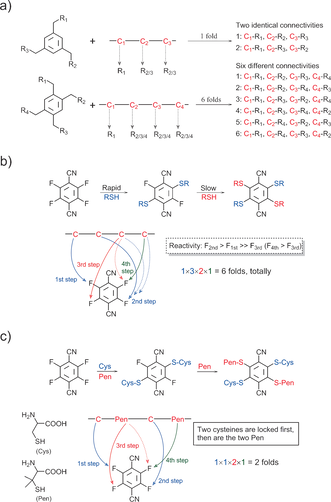
We report the discovery of a small phenyl molecule with four isosteric thiolate-reactive groups of sequentially varied reactivity. This molecule was exploited in combination with cysteine/penicillamine thiolates of different nucleophilic reactivity for precisely regulated and efficient locking (PROP-locking) of linear peptides into multicyclic topologies through a one-pot reaction. The PROP-locking relies on multistep and sequential thiolate/fluorine nucleophilic substitutions, which is not only rapid but highly specific, thus enabling rapid locking of peptides with high amino acid diversities without protecting groups. Several tricyclic peptide templates and bioactive peptides were designed and synthesized using the PROP-locking strategy. We believe that tricyclic peptides precisely locked through stable thioether bonds should be promising structurally constrained scaffolds for developing potential therapeutics and target ligands.