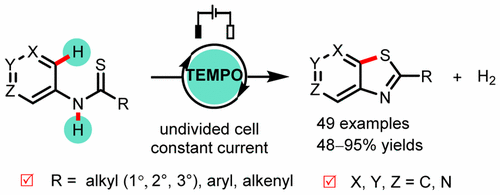
我室徐海超教授在 ACS Catalysis 上发表题为"TEMPO-Catalyzed Electrochemical C–H Thiolation: Synthesis of Benzothiazoles and Thiazolopyridines from Thioamides"的研究论文。
文章链接:http://pubs.acs.org/doi/abs/10.1021/acscatal.7b00426
摘要:
Benzothiazoles and thiazolopyridines are widely prevalent in pharmaceuticals and organic materials. Herein, we report a metal- and reagent-free method for the uniform synthesis of benzothiazoles and thiazolopyridines through 2,2,6,6-tetramethylpiperidine-N-oxyl radical (TEMPO)-catalyzed electrolytic C–H thiolation. This dehydrogenative coupling process provides access to a host of benzothiazoles and thiazolopyridines from N-(hetero)arylthioamides. Mechanistic studies suggested that the thioamide substrate was oxidized with the electrochemically generated TEMPO+ through an inner-sphere electron transfer to afford a thioamidyl radical, which undergoes homolytic aromatic substitution to form the key C–S bond.