我室董全峰教授在 ACS Catalysis 上发表论文:A Synergistic Catalytic Mechanism for Oxygen Evolution Reaction in Aprotic Li-O2 Battery
文章链接:https://pubs.acs.org/doi/10.1021/acscatal.8b02236
摘要:
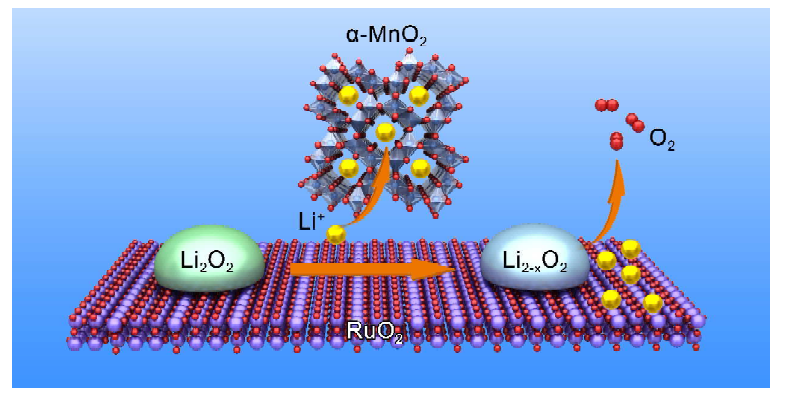
The large polarization of a Li-O2 battery is derived from oxygen evolution reaction (OER) processe. To achieve a long-life Li-O2 battery with high round-trip efficiency, various catalysts have been extensively investigated for oxygen cathodes, especially for OER processes. Here, we designed an in-situ growth of α-MnO2/RuO2 composite on graphene nanosheet with carbon embedded structure as the cathode electrode for Li-O2 battery. The synergistic catalytic effect between the α-MnO2 and RuO2 has significantly improved the OER kinetics. The fabricated Li-O2 battery can deliver a high reversible capacity of 2895 mAh/gcomposite with a low charge overpotential of 0.25 V (0.34 V lower than bare RuO2 cathode). The results revealed that more LiO2 intermediates formed when α-MnO2 was introduced into RuO2 electrode during the oxidation of Li2O2. The facilitation of initial Li extraction was confirmed by density functional theory (DFT) calculations, which shows that the α-MnO2 and RuO2 interfaces can stabilize the primary Li ions and Li2−xO2 intermediates respectively. Subsequently, Li2−xO2 would be easily oxidized to O2 by RuO2 catalyst. With the synergy between α-MnO2 and RuO2, the initial delithiation process and O2 evolution are promoted simultaneously. By combining theoretic and experimental results, we proposed a synergistic catalytic mechanism for the OER processes.