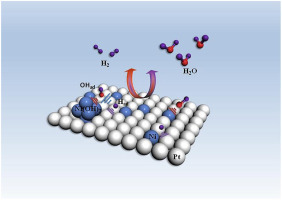
我室孙世刚教授在 Nano Energy 上发表论文:Does the oxophilic effect serve the same role for hydrogen evolution/oxidation reaction in alkaline media?
文章链接:https://www.sciencedirect.com/science/article/pii/S2211285519304513
摘要:
Improving the slow kinetics of hydrogen evolution/oxidation reaction(HER/HOR) on Pt in the alkaline electrolyte is key to the development of water splitting and hydroxide exchange membrane fuel cells, which feature a potential cost advantage over their acid-operating counterparts. However, it is still unconfirmed whether adsorbed surface hydroxyl species (OHad) plays a significant role in determining HER/HOR activity. Moreover, the active sites should be different in the alkaline due to the sluggish reaction rate. In the present work, electrochemical tests have shown that for modified bulk Pt surface and Pt3Ni nanoalloy, HER rate is co-determined by the oxophilic effect and electronic effect, while the rate of HOR is associated with the electronic effect. Density functional theory (DFT) calculations reveal the fundamentally different HER and HOR mechanism of Pt-based nanoparticles, and the surface charge may account for such difference. Finally, the adsorption and oxidation of carbon monoxide (CO) as a novel descriptor are provided to predicate the activity of HER and HOR.