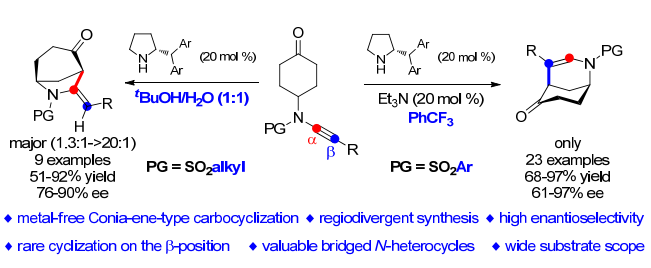
我室叶龙武教授在 ACIE 上发表论文:Organocatalytic Enantioselective Conia‐Ene‐Type Carbocyclization of Ynamide‐Cyclohexanones: Regiodivergent Synthesis of Morphans and Normorphans
文章链接:https://onlinelibrary.wiley.com/doi/10.1002/anie.201908495
摘要:
Catalytic carbocyclization of alkynyl carbonyls has attracted considerable interest in organic synthesis because of its high bond‐forming efficiency and atom economy in the formation of functionalized cyclic compounds. However, examples of such an asymmetric version are quite scarce, and have so far been limited to transition metal catalysts. Described herein is an organocatalytic enantioselective desymmetrizing cycloisomerization of arylsulfonyl‐protected ynamide‐cyclohexanones, which represents the first metal‐free asymmetric Conia‐ene‐type carbocyclization. This method allows the highly efficient and atom‐economical construction of a range of valuable morphans with wide substrate scope and excellent enantioselectivity (up to 97% ee). In addition, such a cycloisomerization of alkylsulfonyl‐protected ynamide‐cyclohexanones can lead to the divergent synthesis of normorphans as the main products with high enantioselectivity (up to 90% ee). Moreover, theoretical calculations are employed to elucidate the origins of regioselectivity and enantioselectivity.