我室霍浩华教授在 Nature communications 上发表论文:Asymmetric benzylic C(sp3)−H acylation via dual nickel and photoredox catalysis
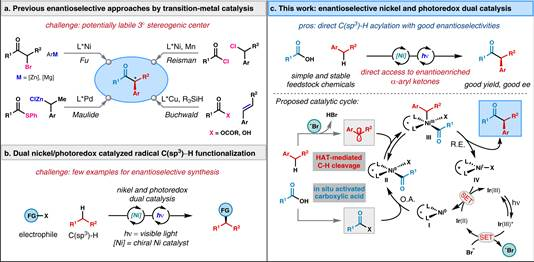
摘要:Asymmetric C(sp3)−H functionalization is a persistent challenge in organic synthesis. Here, we report an asymmetric benzylic C−H acylation of alkylarenes employing carboxylic acids as acyl surrogates for the synthesis of α-aryl ketones via nickel and photoredox dual catalysis. This mild yet straightforward protocol transforms a diverse array of feedstock carboxylic acids and simple alkyl benzenes into highly valuable α-aryl ketones with high enantioselectivities. The utility of this method is showcased in the gram-scale synthesis and late-stage modification of medicinally relevant molecules. Mechanistic studies suggest a photocatalytically generated bromine radical can perform benzylic C−H cleavage to activate alkylarenes as nucleophilic coupling partners which can then engage in a nickel-catalyzed asymmetric acyl cross-coupling reaction. This bromine-radical-mediated C−H activation strategy can be also applied to the enantioselective coupling of alkylarenes with chloroformate for the synthesis of chiral α-aryl esters.
文章链接:https://www.nature.com/articles/s41467-021-23887-2