The materials that we depend on rely upon ever-increasing structural complexity for their function. The use of chemical self-assembly as a synthetic technique can simplify materials preparation by shifting intellectual effort away from designing molecules, and towards the design of chemical systems that are capable of self-assembling in such a way as to express desired materials properties and functions. Below are shown the subcomponent precursors and structures of three of products that can form functional constituents of these systems (Fig.1).
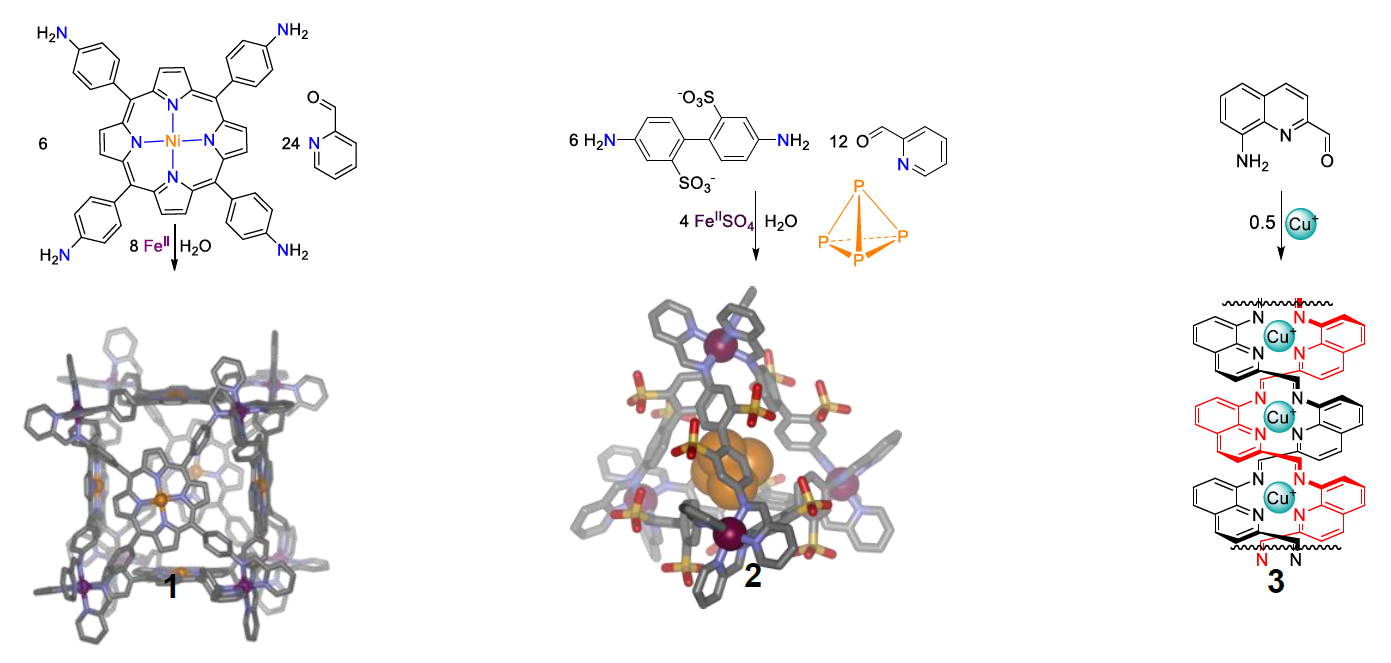
Fig. 1 FeII8 cubiccage 1,1 FeII4 tetrahedral cage 2,2 and electroluminescent CuIn double-helical polymer 33
Current challengesinvolve inducing multiple structures to form in parallel,4 such that they may act in concert to achieve a catalytic goal,5 our techniques allow entry into the emerging field of systems chemistry.6 Functional systems that we have recently developed include afuel-controlled self-assembly process7 and a triphasic sorting system, wherein three guests are selectivelyencapsulated within three cages, each in turn soluble in only one of threemutually-immiscible phases (water and two different ionic liquids).8
1. W. Meng, B.Breiner, K. Rissanen, J.D. Thoburn, J.K. Clegg, J.R. Nitschke, Angew. Chem. Int.Ed.2011, 50, 3479.
2. P. Mal, B. Breiner, K. Rissanen, J.R. Nitschke, Science2009, 324, 1697.
3. J.L.Greenfield, F.J. Rizzuto, I. Goldberga, J. Nitschke, Angew. Chem. Int. Ed.2017,DOI: 10.1002/anie.201702320.
4. A. Jiménez,R.A. Bilbeisi, T.K. Ronson, S. Zarra, C. Woodhead, J.R. Nitschke, Angew. Chem. Int. Ed.2014, 53, 4556.
5. A.G. Salles, S.Zarra, R.M. Turner, J.R. Nitschke, J. Am.Chem. Soc.2013, 135, 17052.
6. J.R. Nitschke, Nature2009, 462, 736.
7. C.S. Wood, C.Browne, D.M. Wood, J.R. Nitschke, ACSCent. Sci.2015, 1, 504.
8. A.B. Grommet, J.L. Bolliger,C. Browne, J.R. Nitschke, Angew. Chem.Int. Ed.2015, 54, 15100.